Amphetamines
Amphetamines are a class of compounds that includes amphetamine itself and its derivatives. Many amphetamines have notable psychoactive properties and are common drugs. Some of them are also of limited use in medicine in the treatment of ADHD and narcolepsy.
Examples of amphetamine derivatives are methamphetamine, ephedrine, cathinone, methcathinone, 3,4-methylenedioxyamphetamine (MDA), 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”), 2,5-dimethoxy-4-bromoamphetamine (DOB).
History
Although the basic compound of the class, amphetamine, was obtained by synthesis at the end of the XIX century, some of its derivatives are found in nature and have a long history of use. Ephedra has been used in China as a medicinal plant for 5,000 years. Its active components are the alkaloids ephedrine, pseudoephedrine, norephedrine (phenylpropanolamine) and norpsevdoephedrine (katin). In Yemen and Ethiopia, there has long been a tradition of chewing kata leaves in order to achieve a stimulating effect. The active substances of kata are cathinone and, to a lesser extent, katin (norpsevdoephedrine).
Amphetamine was first synthesized in 1887 by the German chemist L. Edeleano in the form of a racemic mixture of right-rotating and left-rotating enantiomers. The substance did not attract much attention to itself then. In 1912 (according to other sources — in 1914) MDMA was synthesized for the first time as an intermediate product, which also went unnoticed. In the 1920s, in the process of searching for ways to synthesize ephedrine, which was already used to treat asthma, but was extracted exclusively from natural sources, the right-handed isomer of amphetamine (dextroamphetamine, D-amphetamine), as well as methamphetamine, was synthesized in its pure form. The beginning of the use of amphetamines in medicine was laid by the pharmaceutical company Smith, Kline & French (now part of GlaxoSmithKline) in the early 1930s, offering it to the market as a remedy for the common cold (decongestant) called “Benzedrine”. Subsequently, amphetamine began to be used in the treatment of narcolepsy, parkinsonism and depression. The stimulating effects of amphetamines were quickly discovered, and the abuse of amphetamines was practiced as early as 1936.
During the Second World War, amphetamines were used by the armed forces of the belligerents to help personnel go without sleep for a while.
Large-scale abuse of amphetamines began in post-war Japan and spread relatively quickly to other countries. Since the 60s, modified (“designer”) amphetamines, such as MDA and PMA, began to gain popularity. MDMA was rediscovered and popularized by the American chemist Alexander Shulgin in 1965, after which it was used for some time in psychotherapy sessions. In 1970, the “Controlled Substances Act” was adopted in the United States, placing amphetamines in Schedule II, which limited their non-medical use. The “street” use of MDMA was recorded as early as 1972. In 1985, MDMA was listed by the American authorities in List I, which meant an almost complete ban on its use.
Since the mid-1990s MDMA (“Ecstasy”) it is becoming a popular drug among young people, widely used at raves. Quite often, substances other than MDMA are sold under the guise of “Ecstasy”.
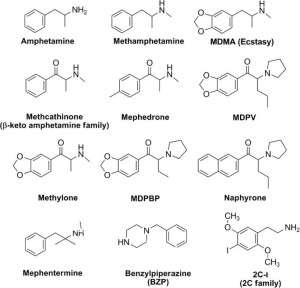
Building
Amphetamines are a subgroup of phenylethylamine derivatives. The base compound of the class is actually amphetamine or α-methylphenylethylamine. Due to the substitution of hydrogen atoms, a large number of compounds can be obtained. Methylation (less often — ethylation) of the amino group and substitutions by the phenyl group are quite typical:
| Substance | N | α | β | 2(Phenyl group) | 2(Phenyl group) |
| Amphetamine (α-methylphenylethylamine) | −CH3 | ||||
| Methamphetamine (N-methylamphetamine) | −CH3 | −CH3 | |||
| Ephedrine, pseudoephedrine | −CH3 | −CH3 | −OH | ||
| Cathinone (norephedrone) | =O | ||||
| Methcathinone (ephedrone) | −CH3 | −CH3 | =O | ||
| MDA (3,4-methylenedioxyamphetamine) | −CH3 | −O−CH2−O− | |||
| MDMA (3,4-methylenedioxymethamphetamine) | −CH3 | −CH3 | −O−CH2−O− | ||
| MDEA (3,4-methylenedioxy-N-ethylamphetamine) | −CH2−CH3 | −CH3 | −O−CH2−O− | ||
| MDMC (methylone, 3,4-methylenedioxy-methcathinone) | −CH3 | −CH3 | =O | −O−CH2−O− | |
| MBDB (N-methyl-1-(3,4-methylenedioxyphenyl)-2-aminobutane) | −CH3 | −CH2−CH3 | −O−CH2−O− | ||
| PMA (para-methoxyamphetamine) | −CH3 | ||||
| PMMA (para-methoxymethamphetamine) | −CH3 | −CH3 | |||
| 4-MTA (4-methylthioamphetamine) | −CH3 | ||||
| 3,4-DMA (3,4-dimethoxyamphetamine) | −CH3 | −O−CH3 | |||
| 3,4,5-TMA (3,4,5-trimethoxyamphetamine, α-methylmescaline) | −CH3 | −O−CH3 | |||
| DOM (2,5-dimethoxy-4-methylamphetamine) | −CH3 | −O−CH3 | |||
| DOB (2,5-dimethoxy-4-bromoamphetamine) | −CH3 | −O−CH3 |
Physiological effect
Classification of central effects
The central action of amphetamines is diverse. There are three main classes of effects caused by amphetamines:
psychostimulating,
hallucinogenic,
empathogenic — creates a feeling of well-being, openness, emotional closeness to other people (empathy).
Various amphetamines can cause these actions either alone or in combination.
Animal studies have shown that these classes of effects are independent. At the same time, from the point of view of symptoms, this classification is conditional, since, for example, amphetamine is also capable of producing hallucinations at high doses (“amphetamine psychosis”).
The stimulating effect of amphetamines is similar to the effect of cocaine, but has a longer duration of action.
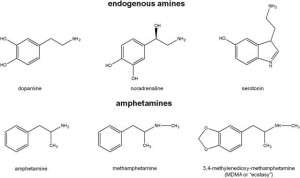
Mechanisms
The mechanism of the stimulating effect of amphetamines is mainly associated with an increase in the release of catecholamines (norepinephrine and dopamine). There are two pools of neurotransmitters in the presynaptic termination: vesicular and cytoplasmic. During normal synapse operation, catecholamine release is carried out by exocytosis of vesicles containing a neurotransmitter. In the future, the neurotransmitter is reuptake from the synaptic cleft into the cytoplasm of the neuron, carried out by the membrane transporter of catecholamines. From the cytoplasm, the neurotransmitter penetrates back into the vesicles due to the work of the vesicular monoamine transporter (VMAT).
In the presence of amphetamine or its analog, the direction of action of membrane transporters and VMAT is inverted. The neurotransmitter gets from the vesicles into the cytoplasm, and from the cytoplasm into the synaptic cleft. As a result, the vesicles are emptied, the vesicular release of the neurotransmitter decreases, and the concentration of the neurotransmitter in the synaptic cleft increases. At the same time , three mechanisms are involved:
At low concentrations, amphetamines affect only the membrane transporters of catecholamines, causing an inversion of the direction of neurotransmitter transport (from the cytoplasm to the synaptic cleft). This effect is partly due to the fact that amphetamines, whose molecular structure is close to that of endogenous catecholamines, penetrate the cytoplasm of the neuron through membrane transporters, which causes the exchange diffusion of the neurotransmitter in the opposite direction. Another reason for the reverse transport of catecholamines from the cytoplasm to the synaptic cleft is the increase in the intracellular concentration of Na+ ions caused by amphetamines.
At medium concentrations, in addition to the previous mechanism, another one is involved: amphetamines that have penetrated the cytoplasm of the neuron interact with the vesicular monoamine transporter (VMAT), whose normal function is to maintain a high concentration of the neurotransmitter inside the vesicle by capturing it from the cytoplasm and moving it inside the vesicle. Amphetamines cause the reverse movement of the neurotransmitter from the vesicle to the cytoplasm, due to which the concentration of the neurotransmitter in the cytoplasm increases significantly, and through the mechanism described above, it enters the synaptic cleft. The mechanism of interaction of amphetamines with VMAT is similar to the mechanism of their interaction with membrane transporters.
At high concentrations, another mechanism is involved associated with the penetration of amphetamines into the vesicles through interaction with the vesicular transporter of monoamines, which causes alkalinization of the contents of the vesicles and, as a consequence, an additional release of the neurotransmitter into the cytoplasm.
The mechanism of the stimulating effect of amphetamine and its analogues. On the left — the normal mode of operation of the dopaminergic terminal, on the right — in the presence of amphetamine: the vesicles are emptied, the vesicular release of dopamine decreases, the contents of the vesicles are ejected into the cytoplasm and further into the synaptic cleft. DAT is a dopamine transporter, VMAT is a vesicular monoamine transporter.
Monoamine reuptake inhibitors, including cocaine, reduce the release of monoamines caused by amphetamine stimulants.
The effect of hallucinogenic amphetamines is similar to that of other classical hallucinogens and is associated with their agonism to serotonin receptors of type 5-HT2A. Empathogenic effects are associated with stimulation of serotonin release, the mechanisms of which are similar to the mechanisms of stimulation of catecholamine release described above.
The relationship between structure and action
There is a relationship between the structure of the compound and the effects it causes. Substitutions of the amine group enhance the stimulating effect of the substance, but may reduce other actions. Examples are methamphetamine and N-hydroxyamphetamine (one of the metabolites of amphetamine), which are more powerful stimulants than amphetamine.
The replacement of the methyl group in the α position with an ethyl group leads to a decrease in stimulating and hallucinogenic effects, but does not affect the empathogenic effect (as in MBDB) and appetite suppression (an example of the latter is phentermine). At the same time, the removal of the methyl group in the α position, as a rule, leads to a decrease in the effectiveness of the substance: for example, phenylethylamine does not penetrate the blood-brain barrier well and is rapidly metabolized.
Substitutions in the phenyl group lead to a decrease in the stimulating effect, but other effects may appear. Substitution in position 4 is usually associated with serotonergic action.
The right-handed enantiomers of amphetamines (such as dextroamphetamine) are usually 4-10 times more effective than the left-handed ones. One of the exceptions is MDMA and some related substances, in which the efficacy does not depend on the isomer.
Peripheral action
The effect of amphetamines on the autonomic nervous system is mainly associated with the release of norepinephrine, which results in increased stimulation of α- and β-adrenergic receptors, which can lead to tachycardia, high blood pressure, mydriasis (pupil dilation), increased sweating and hyperthermia.
Toxicity
Acute toxicity of amphetamines is primarily associated with their effect on central and peripheral adrenoreceptors. On the part of the central nervous system, it can manifest itself in the form of psychoses, visual and tactile hallucinations, anxiety states. On the part of the cardiovascular system, tachycardia and high blood pressure are frequent manifestations. The immediate causes of death from amphetamine use are, as a rule, hyperthermia, cardiac arrhythmia or intracerebral hemorrhage.
Unlike many other drugs, amphetamine and especially methamphetamine are neurotoxic and can cause irreversible damage to dopaminergic and serotonergic neurons. It is believed that the toxic effect of amphetamines on dopaminergic neurons is associated with the formation of free radicals and peroxynitrite, a strong oxidant. In addition, MDMA in high doses reduces the amount of antioxidants (glutathione and vitamin E) in the brain, which leads to its neurotoxicity. The question of the presence or absence of neurotoxicity of MDMA with prolonged use in normal doses remains a subject of debate.
Dependence
Amphetamines (with the exception of classical hallucinogens) can, with prolonged use, cause severe mental dependence, expressed in an irresistible desire to take the drug. Such symptoms can last up to several weeks. At the same time, even after a long break in use, there is a high probability of relapse. It is believed that mental dependence on amphetamines, as well as on other drugs, is associated with the stimulation of dopaminergic neurons in the ventral region of the tire, responsible for the feeling of reward, which affects the processes of learning and adaptation.
The question of the existence of physical dependence on amphetamine stimulants is ambiguous. When amphetamine stimulants are discontinued after prolonged use (or repeated use for a relatively short time), fatigue, depression, drowsiness and hunger are observed. These symptoms can be considered components of withdrawal syndrome or consequences of the body’s usual reaction to lack of sleep and food, concomitant with chronic use of amphetamine stimulants.
Classical hallucinogens (such as DOM) do not cause addiction. Studies have shown that laboratory animals, easily trained to use stimulants independently, do not use hallucinogens of their own volition.
Forms of release, methods of use and pharmacokinetics
The free bases of amphetamines are liquids with limited stability. For this reason, amphetamines are distributed in the form of salts (sulfates, phosphates and hydrochlorides). As a rule, amphetamine is marketed in the form of tablets (less often capsules or syrups), methamphetamine — in the form of powder for inhalation or preparation of a solution for intravenous administration (less often in the form of tablets or capsules). Crystal methamphetamine hydrochloride (“ice”), intended for smoking, is also widespread. The main form of methylenedioxyamphetamines (MDA, MDMA, MDEA) are tablets with logos.
Amphetamines are characterized by good bioavailability for oral use, and they can also be used intranasally. Crystal methamphetamine hydrochloride (“ice”) is smoked. Drug addicts with experience inject amphetamines intravenously. The lipophilicity of amphetamines allows them to easily overcome the blood-brain barrier. A typical oral dose is 5-20 mg for amphetamine and methamphetamine, 80-150 mg for MDA and MDMA, 3-10 mg for DOM and 1-3 mg for DOB. The half-life is 8-30 hours for amphetamine, 12-34 hours for methamphetamine and 5-10 hours for MDMA. Unchanged, 30% of amphetamine, 40-50% of methamphetamine and 65% of MDMA are excreted.
The duration of action of amphetamines is usually about 4-6 hours, in some cases up to 24 hours.
Treatment of amphetamine poisoning
One of the most dangerous manifestations of an overdose of amphetamine stimulants is hyperthermia and arousal associated with amphetamine-induced delirium. These factors can contribute to rhabdomyolysis — necrosis of skeletal muscles. Therefore, the primary measures in case of an overdose of amphetamine stimulants are immobilization of the patient with the help of physical means (in order to prevent the possibility of harming the patient to himself or others) and sedation by intravenous administration of sedatives. The most suitable class of drugs for this purpose are benzodiazepines, which have a high therapeutic index and a good anticonvulsive effect. Diazepam is administered intravenously in doses of 10 mg, repeated several times if necessary until the patient completely calms down (the total dose may be more than 100 mg of diazepam). Benzodiazepines are also useful in the case of cocaine overdose, the symptoms of which are similar to those of an amphetamine overdose. Instead of benzodiazepines, dopamine receptor antagonists such as haloperidol or droperidol can be used, which have shown high efficacy in animal studies, but, according to the results of clinical trials, do not have significant advantages over benzodiazepines, while having a higher risk of side effects.
In case of significant hyperthermia, external cooling should be provided. If necessary, intravenous hydration is also used, providing a urine output of about 1-2 ml / kg / h. In the case of high blood pressure, alpha-adrenergic receptor antagonists (such as phentolamine) or vasodilators (sodium nitroprusside, nitroglycerin) are used.
Poisoning with hallucinogens rarely leads to danger to life. In the case of such poisoning, benzodiazepines are also used. With hyperthermia, external cooling is provided. Antipsychotics should be used with caution, as they can cause panic and increase the risk of perception disorders associated with the use of hallucinogens.
Treatment of dependence on amphetamine stimulants
The principles of treatment of amphetamine addiction are similar to the principles of treatment of addictions to other stimulants, for example, cocaine. Since physical dependence on amphetamines is weakly expressed or absent, drug withdrawal is carried out simultaneously. It is generally believed that gradual dose reduction, sometimes used in opioid addiction, is ineffective in relation to dependence on stimulants, although there are preliminary results indicating the possible effectiveness of substitution therapy using dextroamphetamine in the treatment of amphetamine addiction. If the patient has strong arousal, benzodiazepines are used, antipsychotics are used for psychotic symptoms.
The main problems are the fight against mental addiction and the prevention of relapses. The main causes of relapses are psychological problems, such as depression caused by drug withdrawal, boredom, pressure from acquaintances and the pleasure derived from taking the drug. The fight against addiction involves individual work with the patient and the patient’s desire to quit taking drugs.
Despite the ongoing intensive search for drugs that would help fight amphetamine addiction, as of 2008 there are no drugs that would be officially approved for this purpose. Although the tests are far from complete, certain positive results have been achieved with the use of the following drugs:
dextroamphetamine, methylphenidate and modafinil (stimulants);
γ-vinyl-GABA;
bupropion (antidepressant);
risperidone (antipsychotic);
rivastigmine (acetylcholinesterase inhibitor);
lobelin (dopamine reuptake inhibitor).
Synthesis
Most amphetamines are synthetic compounds. Among the exceptions are the cathinone contained in the leaves of kata, as well as ephedrine and pseudoephedrine, the active components of ephedra. These natural alkaloids have a noticeably weaker effect than amphetamine.
From phenyl-2-propanone (P2P) and its derivatives
Amphetamine and its derivatives can be obtained by reducing phenyl-2-propanone (phenylacetone, P2P). There are two common ways of such recovery:
Leucart reaction: interaction of P2P with formamide or ammonium formate (for the synthesis of amphetamine) or N-methylformamide (for the synthesis of methamphetamine) in the presence of formic acid, followed by hydrolysis with hydrochloric acid.
Reductive amination of P2P in the presence of ammonia (for the synthesis of amphetamine) or methylamine (for the synthesis of methamphetamine) using metal catalysts. According to the type of catalyst used , these reactions can be further divided into three types:
Heterogeneous catalytic reduction using platinum oxide, palladium on activated carbon or nickel Renei;
Recovery by amalgams of aluminum, zinc or magnesium.
Reduction by metal hydrides, such as lithium alumohydride or sodium borohydride.
P2P itself is most often obtained by the reaction of phenylacetic acid with acetic anhydride.
Similarly, MDA or MDMA can be obtained from 3,4-methylenedioxyphenyl-2-propanone (MDP2P). At the same time, MDP2P itself can be obtained from piperonal.
In Russia, P2P and MDP2P are included in the list of narcotic drugs, psychotropic substances and their precursors, the turnover of which is prohibited (list I); phenylacetic acid, acetic anhydride and piperonal are listed in the list of precursors of narcotic drugs, the turnover of which is limited (list IV).
From ephedrine and its analogues
Methamphetamine can be obtained by reducing ephedrine using one of the following procedures:
conversion of ephedrine to chloroephedrine using thionyl chloride (SOCl2) followed by catalytic hydrogenation (palladium or platinum is most often used as catalysts);
Methamphetamine from ephedrine via chloroephedrine ru.svg
reduction of ephedrine by hydroiodic acid in the presence of red phosphorus.
Methamphetamine from ephedrine with HI ru.svg
Ephedrine itself can be obtained by extraction from ephedra. In case of illegal synthesis, pseudoephedrine extracted from drugs containing it can be used instead.
Similarly, amphetamine can be obtained from norephedrine (phenylpropanolamine).
Metcathinone (ephedron) it can be obtained by oxidation of ephedrine, while potassium permanganate, sodium dichromate or chromium trioxide are most often used as an oxidizer. The reaction takes place in the presence of a strong acid. With the same oxidation of phenylpropanolamine (norephedrine), cathinone can be obtained.
From safrol
Safrol is the main component of sassafras essential oil. Structural similarity makes it possible to synthesize MDA, MDMA and other amphetamines containing a methylenedioxyphenyl group from safrol. Many methods are reduced to obtaining halogenated derivatives of safrol (bromosafrol is often used), which later, interacting with methylamine (for MDMA synthesis), forms the final product. Similarly, MDA and MDEA can be obtained:
From benzaldehyde and its derivatives
Synthesis of amphetamines can be carried out by condensation of benzaldehyde with nitroethane in the presence of an amine as a catalyst, followed by reduction (for example, using lithium alumohydride). In the synthesis of amphetamines with phenyl group substitutions, its derivatives are used instead of benzaldehyde. Thus, piperonal is used for MDMA synthesis.

Market
Currently, amphetamines and their analogues find extremely limited use in medicine. Amphetamine, dextroamphetamine, methamphetamine and methylphenidate (ritalin) are used in various countries in the treatment of narcolepsy and attention deficit hyperactivity disorder.
According to estimates of the United Nations Office on Drugs and Crime (UNODC), in 2007, 230 to 640 tons of amphetamine stimulants (amphetamine, methamphetamine, methcathinone and analogues) and 72 to 137 tons of methylenedioxyamphetamines (MDA, MDMA, MDEA) were illegally produced in the world. The global market for amphetamine stimulants is estimated at $65 billion. The production of amphetamines is mainly concentrated near the markets. International trade is mainly intraregional in nature, which allows the goods to cross the least number of state borders. At the same time, the trade in amphetamine precursors is carried out on a global scale. The leader in the production of methamphetamine is North America (mainly the states of California and Oregon), while the first place in the production of amphetamine and methylenedioxyamphetamines (MDA, MDMA, MDEA) belongs to Europe.
In total, according to the UNODC estimates, 15 to 50 million people used amphetamines at least once during 2007, which is 0.4-1.2% of the total population aged 15 to 64 years. In Russia, this share is 0.2-0.7%.
