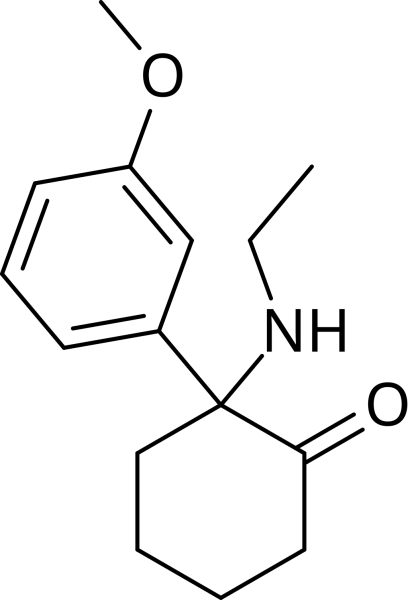
Methoxetamine(MXE)
Methoxetamine, abbreviated MXE, is a dissociative hallucinogen marketed as a designer drug. It differs from many dissociatives such as ketamine and phencyclidine (PCP), which were developed as pharmaceuticals for use as general anesthetics, in that it was developed for distribution in the gray market. Due to its structural similarity to ketamine, it is no longer produced in significant quantities due to near-global bans. This is a rare example of a drug being so widely controlled but not used in medicine.
MXE is an arylcyclohexylamine. It acts primarily as an NMDA receptor antagonist, like other arylcyclohexylamines such as ketamine and PCP.

Effects
MXE is reported to have an effect similar to ketamine. It has often been considered to have opioid properties due to its structural similarity to 3-HO-PCP, but this assumption is not supported by data that show a slight affinity of the compound to the m-opioid receptor. Recreational use of MXE has been associated with hospitalizations due to high and/or combined consumption in the United States and the United Kingdom. Acute reversible cerebellar toxicity was reported in three cases of hospitalization due to an overdose of MXE lasting from one to four days after exposure.
MXE was developed partly in an attempt to avoid the urotoxicity associated with ketamine abuse; it was believed that the increased effectiveness of the compound and the reduced dose would limit the accumulation of urotoxic metabolites in the bladder. Like ketamine, MXE was found to cause inflammation and fibrosis of the bladder after prolonged administration of high doses to mice, although the doses used were quite large. Reports of urotoxicity in humans have not yet appeared in the medical literature.
MXE acts primarily as a selective and high affinity NMDA receptor antagonist, especially with respect to the disocylpine site (MK-801) (Ki = 257 nM). It produces ketamine-like effects. In addition to NMDA receptor antagonism, MXE has been found to act as a serotonin reuptake inhibitor (Ki = 479 nM; IC50 = 2400 nM). On the other hand, it shows little or no effect on the reuptake of dopamine and norepinephrine (K I and IC 50 gt; 10,000 nM). However, MXE has been found to activate dopaminergic neurotransmission, including in the mesolimbic reward pathway. This is a property that it shares with other NMDA receptor antagonists, including ketamine, PCP, and disocylpin (MK-801). Animal studies show that MXE may have a fast-acting antidepressant effect similar to ketamine. A study that evaluated MXE binding at 56 sites, including neurotransmitter receptors and transporters, showed that MXE has K i gt values.; 10,000 nM for all sites except the NMDA receptor dizocylpine site and the serotonin transporter (SERT).
Chemistry
MXE is an arylcyclohexylamine and a derivative of ethycyclidine (PCE). It can also be considered as a beta-keto derivative of 3-methoxyeticyclidine (3-MeO-PCE), or an N-ethyl homologue of methoxmetamine (MXM) and methoxpropamine (MXPr). It is structurally closely related to ketamine and more distantly related to PCP.
MXE hydrochloride is soluble in ethanol up to 10 mg/ml at 25 °C.
Detection in biological fluids
The MXE forensic standard is available, and this compound has been posted on the Forendex website dedicated to potential drugs.
History
The qualitative effects of MXE were first described online in May 2010, and this compound became commercially available in small quantities in September 2010, and by November, the use and sale of MXE had increased so much that it was officially identified by the European Drug Monitoring Center. and drug addiction. By July 2011, the EMCDDA had identified 58 websites selling this compound at a price of 145-195 euros per 10 grams.
Society and culture
Methoxetamine powder.
Media coverage
In January 2012, Mixmag reported that people in the dance and club community had given MXE the slang name “roflcoptr”. Weiss noted that it is likely that this phrase will only be used by “the same politicians, parents, and journalists” who called mephedrone “meow-meow.” After being referred to as mexxy in press releases from the British Home Office, the media adopted that name.
In March 2012, a literature review was published, which examined scientific literature and information on the Internet. It concluded that “the availability of information on new psychoactive drugs such as MXE on the Internet may pose a serious public health problem. More effective levels of international cooperation and new forms of intervention are needed to combat this rapidly growing phenomenon.”
Legal status
Brazil
MXE was classified as a narcotic in Brazil in February 2014.
Canada
As of January 2010, MXE is a controlled substance in Canada.
China
As of October 2015, MXE is a controlled substance in China.
The European Union
On June 16, 2014, the European Commission proposed banning MXE throughout the European Union, subjecting violators to criminal sanctions. This is in line with the risk assessment and control procedure for new psychoactive substances established by the Council: Decision 2005/387/JHA.
Israel
MXE was classified as an illegal drug in Israel in May 2012.
Japan
MXE became a controlled substance in Japan on July 1, 2012, in accordance with an amendment to the Pharmaceutical Act.
Poland
MXE is a controlled substance (Group II-P), which makes it illegal to manufacture, sell or store it in the Republic of Poland since July 1, 2015.
Russia
MXE has been a controlled substance in Russia since October 2011.
Sweden
MXE was classified as a drug in Sweden at the end of February 2012.
Switzerland
MXE has been banned in Switzerland since December 2011.
The United Kingdom
Until March 2012, MXE was not controlled by the UK Drug Abuse Act. In March 2012, the Ministry of the Interior referred MXE to the Advisory Council on Drug Abuse for possible interim control in accordance with the powers given in the 2011 Law on Police Reform and Social Responsibility. The ACMD gave its advice on March 23, with the chairman commenting that “evidence shows that the use of methoxetamine can cause harm to consumers, and the ACMD recommends that it be subject to an interim class-a drug prohibition ordinance.” In April 2012, MXE was placed under temporary drug control, which banned its import and sale for 12 months.
Theresa May commented in her response to ACMD that “the next step in this process is for ACMD to conduct a full assessment of MXE with a view to its ongoing monitoring in accordance with the 1971 Act.” She goes on to say that she hopes ACMD will do this as part of the ketamine review, “including its analogues,” and that this review will be completed “within 12 months of accepting the current order.”
On October 18, 2012, the ACMD released an MXE report stating that “the harm of methoxetamine is commensurate with the Class B of the Misuse of Drugs Act (1971),” despite the fact that the law does not classify drugs based on harm. The report further proposed that all MXE analogues also become Class B drugs, and proposed a comprehensive clause covering both existing and unexplored arylcyclohexamines.
MXE was temporarily banned on February 26, 2013, when it was classified as a Class B drug.
The United Nations
MHE is one of the few substances that, since its adoption, have been under control in accordance with the UN Convention on Psychotropic Substances of 1971. It was listed II in November 2016.
The United States
MXE is not federally registered in the United States, but it is possible that it could be considered a PCE analog, in which case purchase, sale, or possession could be prosecuted under the Federal Analog Law.
In September 2015, a bill was submitted to Congress to include MXE in Schedule I, but the Senate never passed it.
Alabama
MXE is a Schedule I controlled substance in the state of Alabama, making it illegal to buy, sell, or possess in Alabama.
Florida
MXE is a Schedule I controlled substance in the state of Florida, making it illegal to buy, sell, or possess in Florida.
Utah
MXE is a controlled substance in Utah, making it illegal to buy, sell, or possess it in Utah.
